Background on transthyretin amyloidosis
The amyloidoses are a heterogeneous group of disorders characterized by the production of misfolded proteins. These proteins form fibrillar tissue deposits that cause organ injury. In transthyretin amyloidosis (ATTR) fibrils are comprised of either mutated or wild-type transthyretin produced almost exclusively by hepatocytes. Cardiac and peripheral nerve symptoms predominate, but other organs such as the liver, kidneys, and/or GI-tract can also be affected. Liver transplantation in selected patients with familial ATTR has been efficacious but is often accompanied by
post-transplant complications. The rationale for transplant is to replace the organ producing mutated, abnormally-folding transthyretin with one that makes normal protein. Diflunisal and Tafamidis have demonstrated modest
activity in preventing end-organ damage by stabilizing the TTR tetramer. Other agents, such as doxycycline, may interfere with fibril formation. However, to date, we do not have an FDA-approved therapy for ATTR. The article we will be discussing in this journal club activity focuses on a new treatment paradigm, RNA interference (RNAi) therapy to selectively inhibit hepatocyte transthyretin synthesis.
The topic of RNAi was discussed in a previous post. Click HERE if you want to read that for some background on the topic. In that same post, a less detailed overview of the article discussed herein was also provided.
Methodology
- The authors first studied the effects of lipid nanoparticle-encapsulated TTR siRNA administered to cynomolgus monkeys. The transthyretin-lowering effects of single injections of two different RNAi/siRNA preparations (ALN-TTR01 (1.0 mg/kg) and ALN-TTR02 (0.1 mg/kg and 0.3 mg/kg)) were evaluated, as well as multiple doses of ALN-TTR02 (0.3 mg/kg every 4 wks x 5 doses).
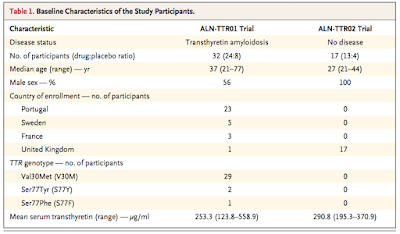
- Sequential multi-center, randomized, single-blind, placebo-
controlled, dose-ranging Phase 1 studies evaluating the
safety and efficacy of a single dose of ALN-TTR01 (dose range: 0.01 mg/kg to 1.0 mg/kg) or ALN-TTR02 (dose range 0.01 mg/kg to 0.5 mg/kg) in people with transthyretin amy-
loidosis or in healthy adult volunteers, respectively. There was a 3:1 drug:placebo randomization. Infusion time: 15-60 minutes. Required pre-medications: dexamethasone, acetaminophen, diphenhydramine or cetirizine, and ranitidine. PK and PD studies were performed. Baseline and post-dosing transthyretin, retinol binding protein (RBP) and Vitamin A levels were assessed. (Rationale for measuring these? The Vitamin A parent compound, retinol, circulates bound to RBP, which in turn is bound to transthyretin....summarized in
mind-numbingdetail HERE). ALso, in a subset of pts with V30M TTR mutation, relative knockdown of mutant TTR compared to normal TTR was compared (remember, pts are generally heterozygous for mutant TTR)
Results
- Monkeys. ALN-TTR01: ~50% TTR knockdown at 7 days (nadir) with recovery to baseline at 28 days. ALN-TTR02: ~75% knockdown of TTR @ day 7 (0.1 mg/kg dose) and ~90% knockdown @ day 14 (0.3 mg/kg dose), with >70% persisting knockdown @ day 28.
- People.
- ALN-TTR01: Statistically significant knockdown of TTR at 1.0 mg/kg dose only (38% reduction, p= 0.01; lower doses and placebo without significant knockdown). Significant inter-patient variability in knockdown. Mutant and normal TTR levels were suppressed to similar degrees. Levels of Vitamin A and RBP seemed to vary with TTR level:
- ALN-TTR02: Significant TTR knockdown with single doses of 0.15 mg/kg or higher in healthy volunteers, persisting out to day 28 post-dose:
- The average TTR reduction following dosing with ALN-TTR02 at doses of at least 0.15 mg/kg was 82-87%.
- Safety: manageable infusion reactions and also cutaneous flushing reactions were seen in a minority of patients. No evidence of thyroid function tests.
Authors' Conclusions
- "Results for ALN-TTR02 were remarkable for the exceptional efficacy compered to ALN-TTR01..." with a safety profile that was "encouraging."
- Amyloidogenic precursor protein level reduction was comparable to that proven to be therapeutically beneficial in other types of amyloidosis (AL and AA types)
- Convincingly demonstrates that RNAi therapy in the form of lipid-encapsulated siRNA can selectively lower mutant and wild-type TTR without significant short-term toxicity.
|
|
- Unclear at this point whether this will translate into improved amyloidosis outcomes (survival benefit, organ function improvement)
- Longterm toxicity needs to be further assessed, particularly with regard to symptoms of Vitamin A deficiency or abnormal thyroid function.
- Platform may be adaptable for treatment of other amyloidoses (though not AL type, since abnormal light chains produced by clonal plasma cells, not hepatocytes).




No comments:
Post a Comment