Here is a synopsis of the article we will be discussing in the December 1st, 2019 session of #amyloidosisJC, an online journal club focusing on all things amyloidosis. The synopsis was prepared by two recipients of the Don Brockman ASH 2019 Trainee Travel Grants supported by the Amyloidosis Foundation, Dr Suresh Balasubramanian (@malignantheme, Karmanos Cancer Institute) and Dr Holly Lee (@holly_dldumls, University of Calgary), with the help of Dr Naresh Bumma (@NB191186, Ohio State University), the faculty co-moderator for the session.
Introduction:
In this round of #AmyloidosisJC, we will be
discussing a clinical paper that discusses the role of minimal (measurable)
residual disease (MRD) in the management of AL amyloidosis.
Here is a link to the paper: Survival impact of achieving minimal residual negativity by multi-parametric flow cytometry in AL amyloidosis by Eli Muchtar et al. 2019 Amyloid.
In this retrospective observation study, the
authors presented updated results with extended follow up of the AL
amyloidosis patients who underwent end of treatment (EOT) MRD assessment by multiparametric flow cytometry (MFC) described in a previous publication in 2017.
Here is a link
to the 2017 paper: The prognostic value of multiparametric flow cytometry in AL
amyloidosis at diagnosis and at the end of first-line treatment. Muchtar E et al. 2017 Blood.
Study objective:
To establish whether
clearance of clonal plasma cells at EOT using sensitive and uniform MFC is
associated with improved OS
Patient population:
Of the original
173 patients with newly diagnosed AL amyloidosis patients (from their 2017
study), 82 patients who had MRD testing at EOT
using sensitive and uniform MFC
were included in this follow up publication.
84% of the patients underwent autologous stem cell transplant as first line therapy (relevance: consider inherent selection bias to include primarily transplant eligible patients who are generally fit and do not have extensive baseline organ involvement
)
Methods:
For MFC testing, reported
sensitivity was 1x10-4 to 2x10-5,
depending on the number of analyzed events, phenotype and DNA index
A
total of 500,000 live cellular events were set as a target per exam (median
gated events achieved 489,922, 25–75% IQR 469,765–493,662)
Results:
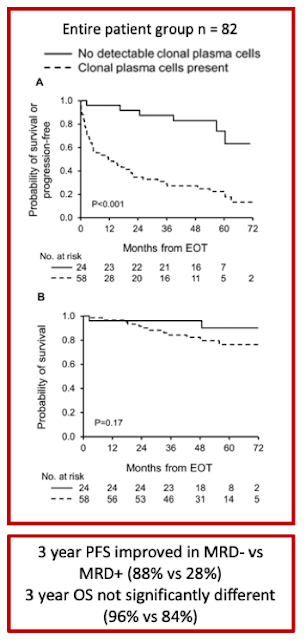
MRD and hematologic response: 29% (24) had negative
MRD, and 71% (58) had positive MRD at EOT. 19.5% (16) had CR, 46.3% (38) had
VGPR, and 28 (34.1%) had less than VGPR
.
Patient outcomes (median 4.6 year follow up):
Among ≥VGPR patients, MRD- was associated with improved PFS compared to patients with MRD+ (3-year PFS 88% vs. 46%, p=0.003), particularly among patients who achieved a complete response (3-year PFS 100% vs. 33%, p=0.001). In contrast, this difference in PFS advantage in VGPR/MRD- compared to those who achieved VGPR/MRD+ did not reach statistical significance (p=0.14)
OS difference was not seen between MRD- and MRD+ groups (3-year OS 96% vs. 84%, p=0.17)
MRD- compared with MRD+ among deep responders was associated with lower level of involved light chain (involved free light chain, median 1.1 vs. 1.7mg/dL; p=0.02) and higher frequency of renal response (100% vs. 68%; p=0.005) . When assessing independent organ function, this difference was not evident in cardiac response
Conclusions:
- Conclusion 1. Despite the retrospective nature of this study with the inherent selection bias to include primarily transplant eligible patients, this work aims at offering novel, robust surrogate endpoint for the design of clinical trials, as well as for optimizing individual patients’ treatment
- Conclusion 2. There may be value in bone marrow biopsy/aspirate at end of therapy in AL amyloidosis patients who achieve VGPR/CR. Presence of MRD is associated with reduced PFS in this group of patients.
- Conclusion 3. The sensitivity of the current standard assay for serum free light chain detection does not discern between MRD positive vs negative among VGPR/CR patients.
- Conclusion 4. This study is notable for presenting one of the largest patient cohorts for end of treatment MRD assessment and applying uniform flow cytometric techniques.
Discussion:
MRD assessment in AL amyloidosis is not yet standardized. Of interest, a study using next generation flow for MRD assessment found that 5 out of 12 MRD positive cases had very low residual tumor burden (<3x10-5), which would have not been detected with lower sensitivity assays (Kastritis et al. 2018). This paper assessed 20 AL patients with hemCR. 8 out of 20 patients were MRD(-). 2 out of 8 patients who had ASCT as primary therapy achieved MRD(-) status, versus 6/12 (50%) patients who did not have ASCT as primary therapy (p = 0.264). In two cases aberrant cells were detected at levels between 10−5 and 10−6 Their reported median sensitivity level of next gen flow was 2.3 x 10−6 (range: 2 × 10−6–3.1 × 10-6)
This is not surprising, as in the myeloma literature, 25% of MRD neg cases by
conventional MFC were found to be MRD positive by next gen flow (Flores-Monteroet al. 2017)
We do not have studies that inform us
how best to manage/ monitor patients deep hematological response based on their
MRD status
- How does MRD status guide frequency of follow up of patients after therapy?
- If a patient initially achieves MRD neg CR but then progresses to MRD positive CR on repeat follow up, does this indicate progression/ requirement for treatment?
- What is the role of transplant in achieving MRD negative status? The impact of daratumumab in achieving MRD negative status? As per our discussion from last week, looking forward to upcoming ISA abstract from @vsanchorawala regarding role of transplant in this topic!
- Does time to MRD achievement matter and if so, what is the optimal time to assess MRD in AL amyloidosis? Interesting report by Muchtar et al. 2019 in Leukemia journal, looked at not only the depth of response (in this case nadir iFLC <2mg/dL) but also the impact of time to nadir iFLC on patient outcomes. Patients whose nadir iFLC occurred after 12 months from EOT had significantly longer PFS and OS compared to patients who reached nadir before 12 months. This raises the question as to whether there a role for MRD assessment not only at EOT but also further out from EOT.
UPDATE 12/1/19 @ 10:19 pm: link to transcript of tonight's twitter discussion CLICK HERE.