This week we will be discussing cardiac staging of AL (light chain) amyloidosis. As mentioned in this post from February 4th, 2018, we are used to the idea of staging AL amyloidosis on the basis of baseline cardiac biomarker measurements. The most widely used system is the European modification of the original 3-stage Mayo 2004 system which uses Troponin T or I and NT-pro-BNP to divide patients into Stages 1, 2, 3a and 3b. The Mayo 2012 system, which incorporates serum free light chain levels, is another 4-stage variation on this theme. Finally, there is a British cardiac staging system for transthyretin (ATTR) amyloidosis which was the subject of a prior #amyloidosisJC discussion. What all of these publications have in common is the use of NT-pro-BNP as a key cardiac biomarker. What all medical centers don't have in common is the ability to order this test without having to send out a blood sample. Many centers use BNP as an alternative. In this installment of #amyloidosisJC, we examine a new 4-stage cardiac staging system for AL amyloidosis devised by researchers at Boston University which incorporates BNP rather than NT-pro-BNP (and we will be lucky enough to have them lead the Twitter discussion!)
Here is a Visual Abstract from the journal Blood summarizing the article:
 |
| Visual Abstract for Lilleness B, et al |
Key Details of the article we will be reviewing:
- A 250-patient derivation cohort from 2016 was used to establish an optimal BNP cut-off to identify cardiac involvement by amyloidosis and also to establish the optimal BNP value to incorporate into a prognostic staging system that corresponded well with the Mayo 2004 system. All the patients in this cohort (except 1) had both BNP and NT-pro-BNP measured. The authors then validated the new staging system with a 1073-patient validation cohort (patients seen between 2004 and 2014, of whom 592 had both BNP and TnI measured at baseline). A Receiver Operating Characteristic (ROC) analysis of 1-yr survival was done to establish a BNP cut-off for BU Cardiac Stage 3b (corresponding to European Cardiac Stage 3b).
- The presence of cardiac involvement was determined by the following criteria, in order of preference: endomyocardial biopsy or cardiac MRI consistent with cardiac amyloidosis, intraventricular septal end-diastole (IVSd) thickness of at least 12 mm obtained on transthoracic echocardiography without other cause of wall thickening (consistent with established consensus criteria), and IVSd at least 11 mm in men or at least 10 mm in women with no history of hypertension or valvular disease (consistent with current reference ranges, as established by the American Society of Echocardiography).
- Based on the ECHO/MRI criteria outlined above, 47% of pts in the derivation cohort had cardiac involvement. Only 22 out of the 116 pts identified as having cardiac involvement (19%) had an endomyocardial biopsy confirming cardiac amyloidosis.
- In deriving the new cardiac staging system, the authors elected to use the same Troponin I (TnI) cut-off of 0.1 ng/mL used in the Mayo 2004 system, and then determine the best BNP cut-off to use with that. The value they found was 81 pg/mL (𝛋 = 0.854 with Mayo 2004 system). 81 pg/mL also turned out to be an optimal value for predicting the presence of cardiac amyloidosis (except for pts with advanced Chronic Kidney Disease, defined as eGFR <30, in whom the BNP and NT-pro-BNP cut-offs were higher).
 |
| Concordance between he BU and Mayo AL cardiac staging systems |
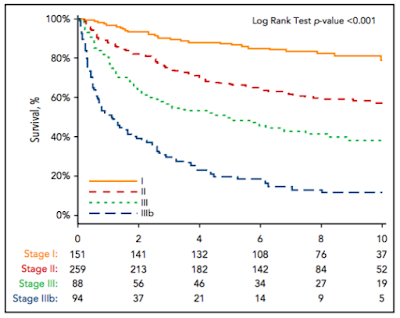
- Of 592 patients with both BNP and TnI levels measured in the validation cohort, 151 patients (25.5%) were assigned to stage I, 259 patients (43.6%) were assigned to stage II, and 182 patients (30.7%) were assigned to stage III. To create a stage IIIb, a BNP threshold of 700 pg/mL was derived by ROC analysis for survival at 1 yr among the 182 pts with stage III disease (AUC, 0.73; 95% CI, 0.66-0.81).
- The median OS from the time of diagnosis was 1.0 year for stage IIIb, 4.3 years for stage III, 9.4 years for stage II, and not reached for stage I patients (Figure 3; HR, 2.6; 95% CI, 1.7-3.9; P , .001). 43.6% of patients with stage IIIb disease died within 6 months.
- The authors comment on the fact that about half of cardiac stage 3 patients die within the first year, after which time the survival curve for this group flattens out. This is similar to what has been noted previously by the Mayo investigators, and it remains a major challenge in the management of AL amyloidosis.
- A BNP-based staging system was hypothesized to be superior to the NT-pro-BNP-based one (since NT-pro-BNP is affected by renal clearance) but this was not confirmed in this work.
- This publication did not address the usage of changes in BNP as a marker of organ response, therefore serial NT-pro-BNP measurement remains a key biomarker of cardiac response (30% reduction indicative of a cardiac response).
Please join us at 9 pm on Wednesday, March 27th, 2019 on Twitter (find us at #amyloidosisJC) for a full and spirited discussion of this article, as well as the other AL cardiac staging papers cited above!